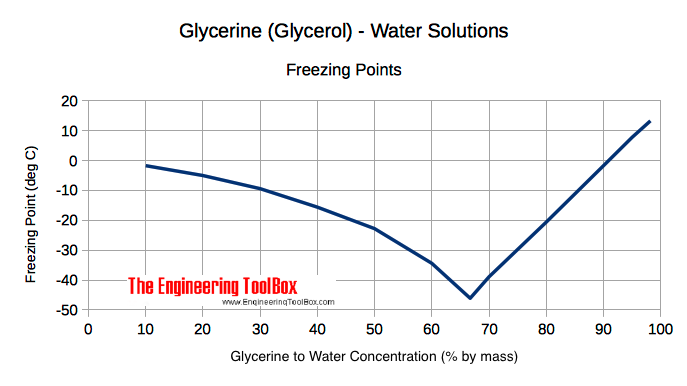
The freezing point of aqueous solution that contains `3%` of urea, `7.45%` KCl and `9%` of glucose - YouTube
What is the freezing point of an aqueous solution containing 10.50 of MgBr2 in 200g of water? - Quora

What should be the freezing point of aqueous solution containing 17g of C(2)H(5)OH is 1000g of water (K(f) for water =1.86 deg kg mol^(-1))?

Freezing point of a 4% aqueous solution of X is equal to freezing point of 12% aqueous solution of Y . If molecular weight of X is A , then molecular weight of Y is:

Calculate the freezing point of an aqueous solution containing `10.5g` of Magnesium bromide in - YouTube

The freezing point of an aqueous solution of `KCN` containing `0.1892 mol Kg^(-1)`, the freezing... - YouTube
The Freezing Points of Aqueous Solutions. IV. Potassium, Sodium and Lithium Chlorides and Bromides | Journal of the American Chemical Society
Q6 Calculate the freezing point of an aqueous solution containing 10.50 g of MaBrz in 200 gof water (molar mass of MgBr2 184 g mol 1 ). Given Kf for water = 1.86 KKg mor

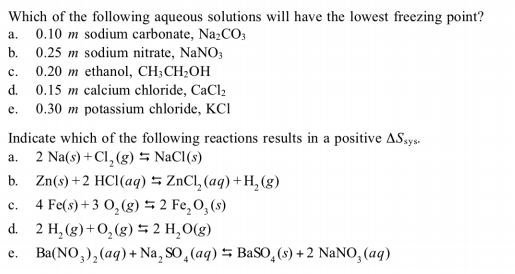
SOLVED: Which aqueous solution will have the lowest freezing point? A. 0.1 m MgCl2 B. 0.1 m NaCl C. 0.1 m Na3PO4 D. pure H2O
The Freezing Points of Aqueous Solutions. X. Dioxane and its Mixtures with Lithium, Sodium and Potassium Chlorides1 | Journal of the American Chemical Society
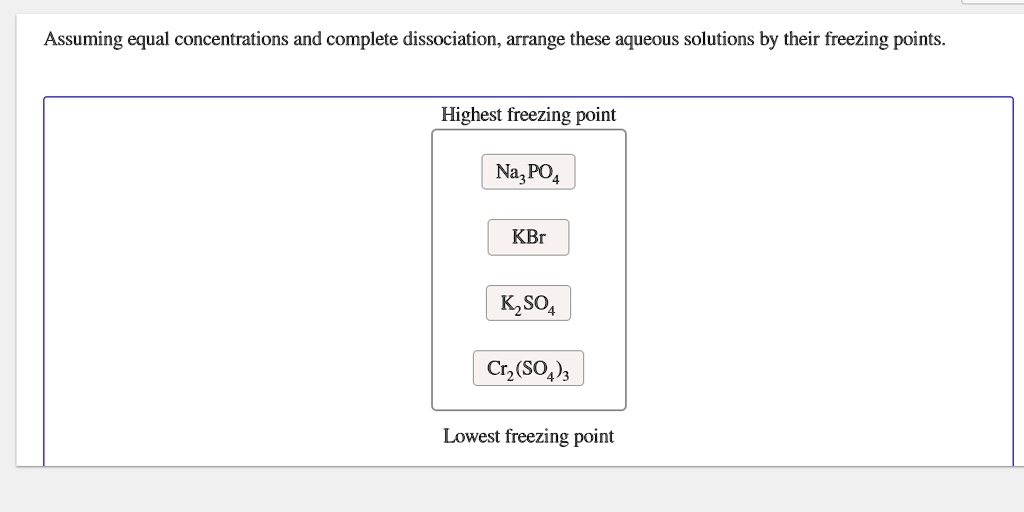
SOLVED: Assuming equal concentrations and complete dissociation, arrange these aqueous solutions by their freezing points. Highest freezing point Na; = PO4 KBr Kz= SO4 Cr2 (SO Lowest freezing point