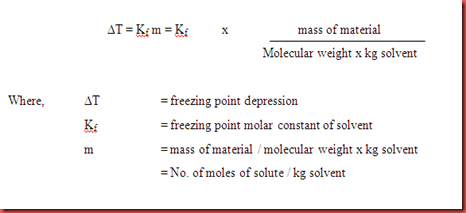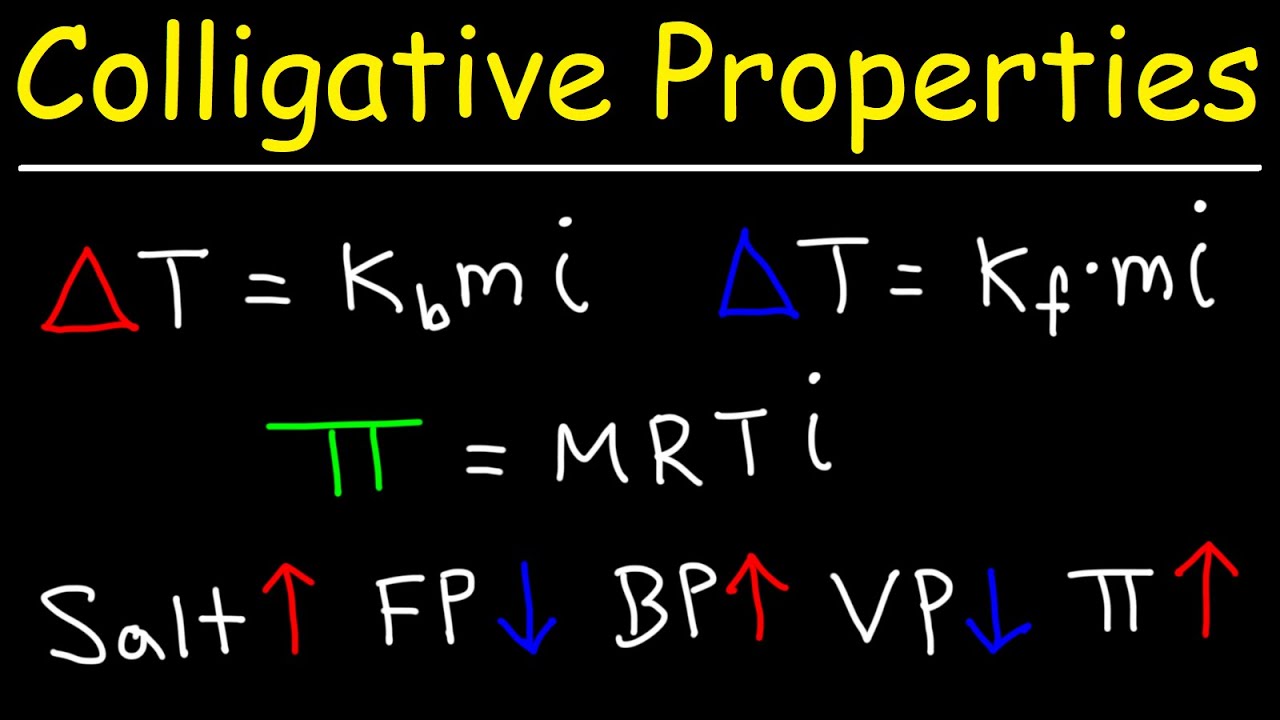
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure - YouTube

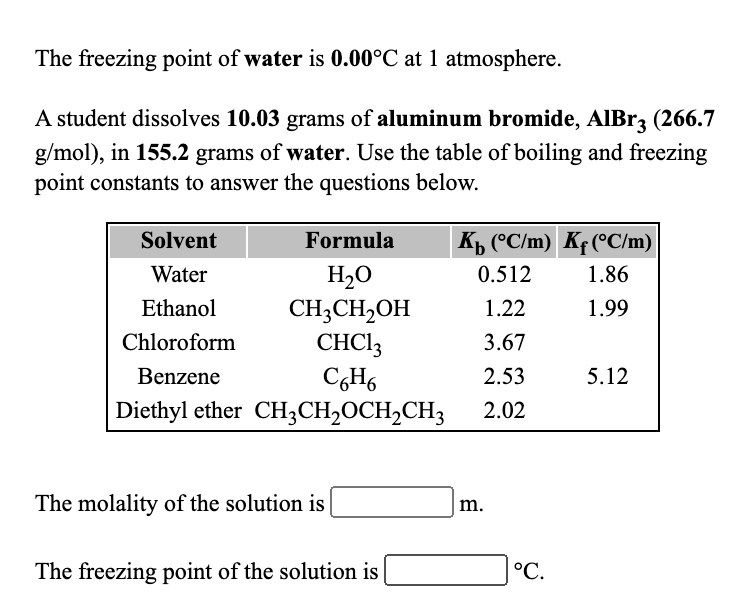
Determine the freezing point depression of H_2O in 1.50 M solution of C_{12}H_{22}O_{11}? | Homework.Study.com

Difference Between Freezing Point Depression and Boiling Point Elevation | Compare the Difference Between Similar Terms
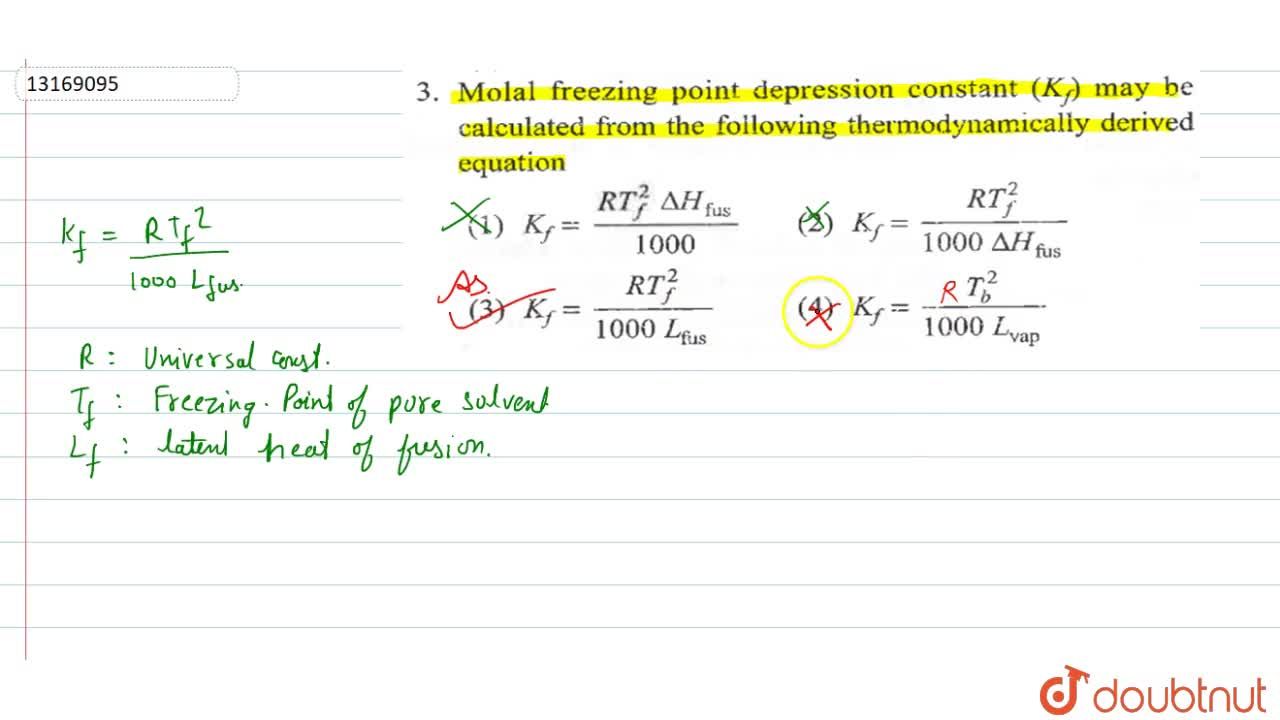
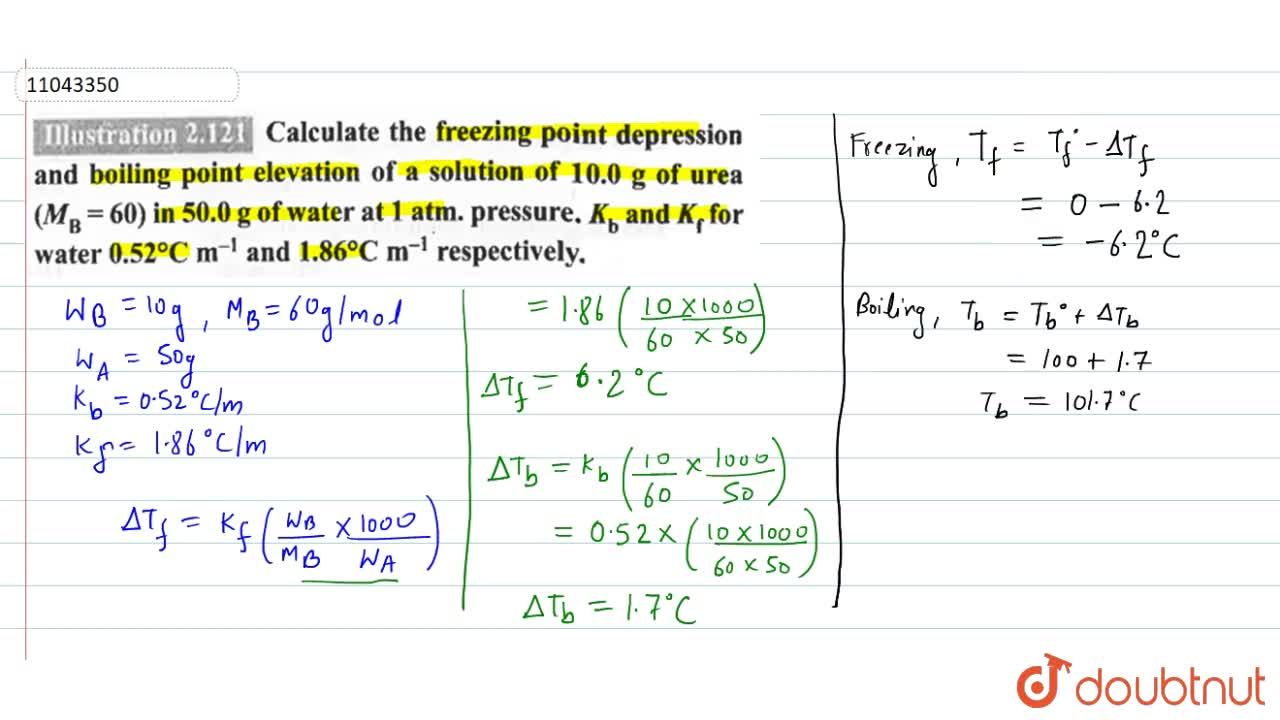
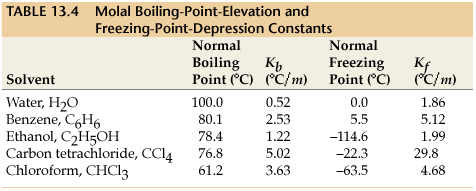
Molal freezing point depression constant (K(f)) may be calculated from the following thermodynamically derived equation

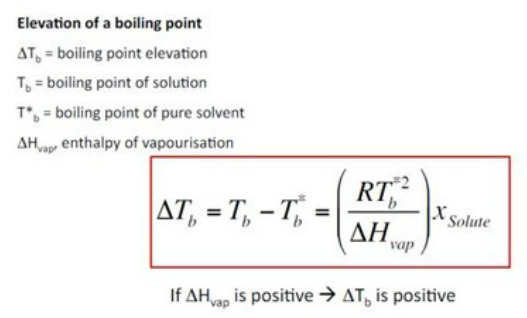
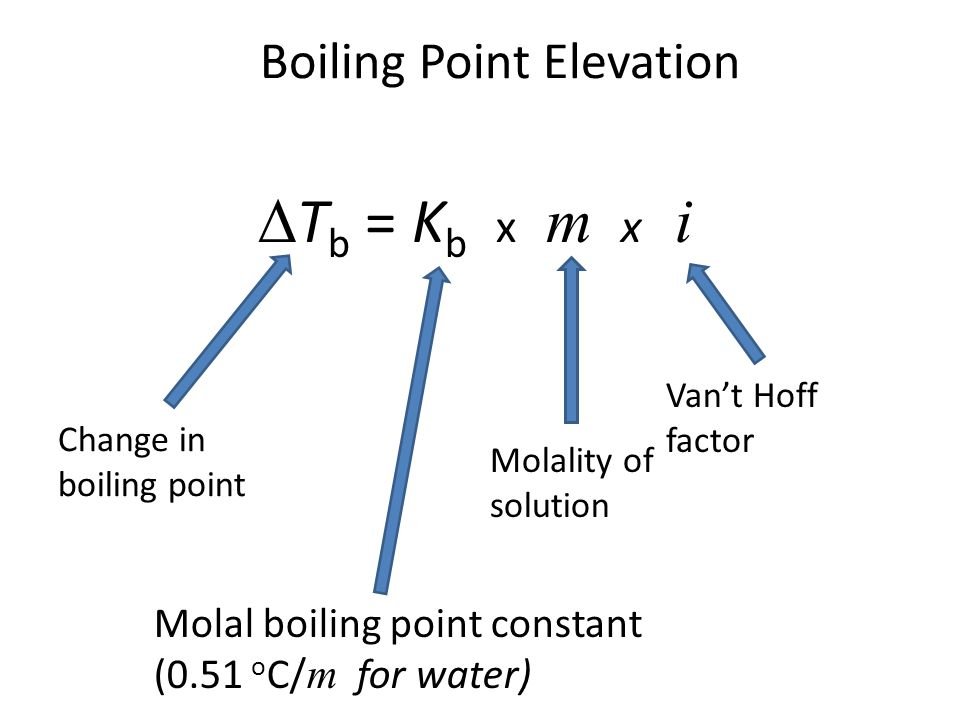
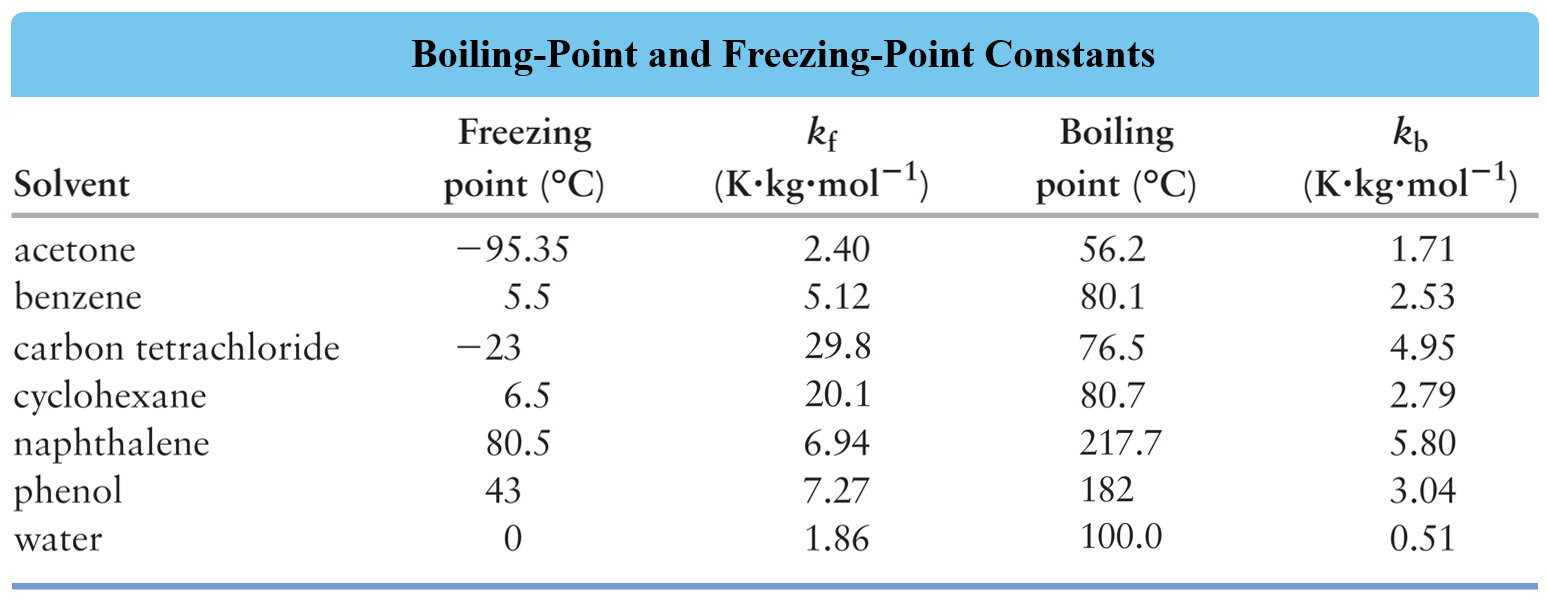
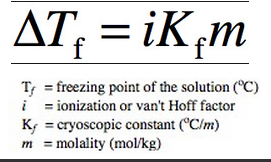
SOLVED: Write the equations relating boiling-point elevation and freezing- point depression to the concentration of the solution. Define all the terms, and give their units.